The FDA Prior Notice Web Entry is a critical step for anyone importing food into the United States. It requires advance electronic notification to the FDA before any food shipment arrives at a U.S. port. This process ensures that imported food undergoes proper regulatory review to protect public health and comply with federal law.Importers use the Prior Notice System Interface to submit detailed information about the shipment, including the type of food, origin, and arrival details. Failure to submit a timely and accurate prior notice can result in shipment delays or refusals. The system is mandatory for all food destined for human or animal consumption unless specifically exempted.Understanding the FDA Prior Notice requirements helps importers navigate the regulatory landscape and avoid costly disruptions. The electronic submission streamlines communication with the FDA and supports the agency’s mission to safeguard the food supply.
Understanding FDA Prior Notice Web Entry
The FDA Prior Notice Web Entry requires specific steps and information to be submitted before imported food shipments reach U.S. ports. It involves detailed data about the product, shipment, and involved parties to ensure compliance with regulations designed to protect public health.
What Is Prior Notice for FDA
Prior Notice is an electronic notification sent to the FDA before food shipments arrive in the United States. It must include detailed information about the shipment, such as the type of food, quantity, origin, and shipment details.This notice allows the FDA to review the shipment for safety risks before it enters the country. It must be submitted through the FDA Prior Notice System Interface (PNSI), which confirms the notice before the shipment’s arrival.The notice must be filed at least four hours before arrival if transported by water or land, or two hours if by air or international mail. This advance submission helps the FDA identify potential risks earlier and take necessary actions.
Types of Products Requiring Prior Notice
Prior Notice applies to all food products intended for human or animal consumption entering the U.S. This includes raw, processed, packaged, and unpackaged foods.
Examples of products requiring Prior Notice include:
- Fresh and frozen fruits and vegetables
- Meat, poultry, and seafood
- Dairy products and eggs
- Baked goods and snacks
- Pet foods and animal feed
The FDA requires notice for all shipments regardless of value or quantity, making it essential for importers to know which products fall under this obligation.
Legal Requirements for US FDA Prior Notice
The requirement to submit Prior Notice is mandated under Title 21 Code of Federal Regulations, Part 1, Subpart I. The law states prior notice must be electronically submitted before the food reaches the first U.S. port.Submission must be within 15 calendar days of arrival but not more than 15 days in advance. The notice must list all relevant details: product description, quantity, manufacturing and shipping facilities, and importer information.Failure to comply can result in refused entry, delays, or possible detention of the food shipment. The FDA also expects accurate and complete data to avoid penalties.
Consequences of Non-Compliance
Failure to submit Prior Notice correctly or on time can lead to significant consequences. Shipments may be held at the port or refused entry entirely, causing costly delays.Penalties can include fines and additional inspections. Repeated violations might lead to more stringent regulatory actions or increased scrutiny on future shipments.Non-compliance risks harm to public safety, which the FDA seeks to prevent by enforcing these requirements strictly. Importers must ensure prior notice is accurate, timely, and complete to avoid disruptions.
How to File US FDA Prior Notice Online
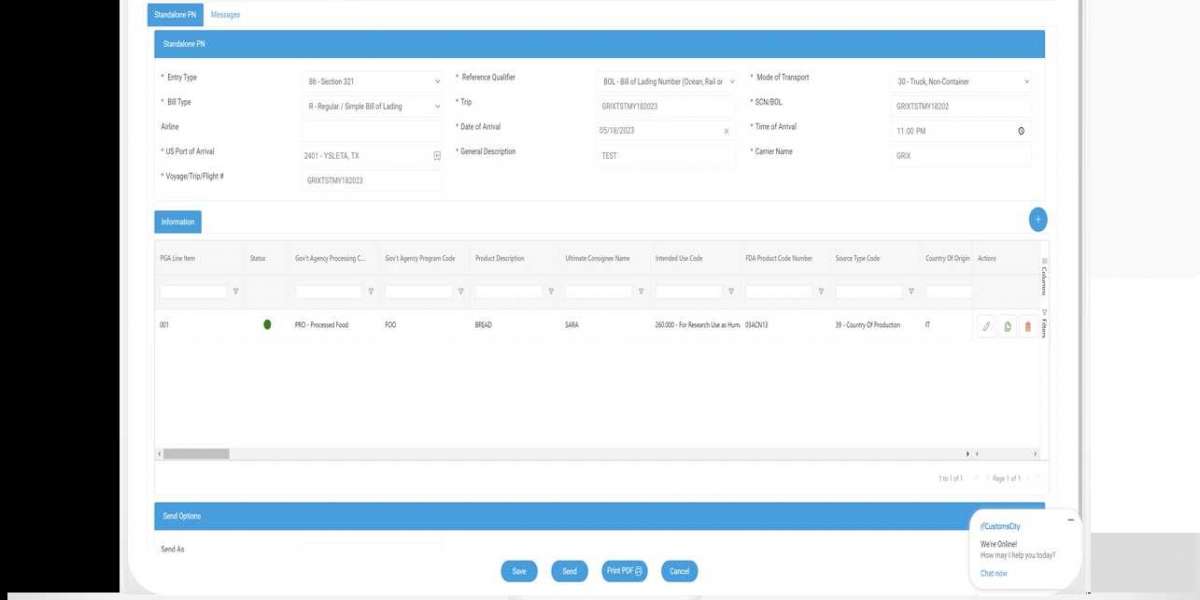
Filing US FDA Prior Notice online requires using the FDA Prior Notice System Interface (PNSI). The process involves account login, detailed data entry, and meeting specific timing requirements depending on the shipment mode.Users must provide precise shipment information and food product details to avoid delays or refusals. It is essential to understand the required data and common pitfalls to ensure smooth submission.
Step-by-Step Filing Process
The process begins by logging into the FDA Prior Notice System Interface (PNSI) with a valid account.Next, the user selects "Create New Web Entry" to start a submission. If the prior notice was partially completed before, the user can retrieve it from "Find Existing."After entering the shipment and product details, the system will prompt a review before generating a confirmation number.
This confirmation must be available to carriers at the port of arrival.
Submission deadlines vary by transportation method:
- Land: at least 2 hours prior
- Air or rail: at least 4 hours prior
- Sea: at least 8 hours prior
Late submissions may lead to refusal or enforcement actions.
Required Information for Web Entry
The web entry requires detailed data about the shipment and the food product.
Important fields include:
- Importer and consignee information
- Product description, including common name and brand
- Quantity and packaging details
- Manufacturer and country of origin
- Facility where the food was processed
Accurate information helps FDA assess risk and plan inspections efficiently.The system accepts only electronically submitted prior notice and confirms receipt before shipment arrival.For shipments sent by international mail, prior notice must be submitted before mailing.
Common Errors and How to Avoid Them
Errors often occur when essential fields are incomplete or incorrectly filled.
Common mistakes include:
- Missing or inaccurate shipment arrival time
- Incorrect product descriptions or codes
- Failure to input correct importer or manufacturer details
- Submitting prior notice too early or late
To avoid errors, users should double-check all information before submission.Keeping the prior notice confirmation number handy prevents complications during shipment inspection.Using the automated entry system carefully and adhering to FDA's timing rules reduces the risk of delays or refusals.